Abstract
Multiple myeloma is an incurable malignancy of terminally differentiated B-cells - also known as plasma cells. These malignant cells are extremely reliant on the bone marrow microenvironment for their growth and survival, as well as their acquired ability to resist therapeutic intervention. Consequently, maintaining primary myeloma cells in vitro remains a challenge. Patients suffering from this incurable disease often develop osteolytic lesions, due to an imbalance between osteoblasts and osteoclasts, which cause bone pain and a high frequency of fractures. This project aims to create a physiologically relevant in vitro model of myeloma that incorporates an osteoclast microenvironment. Osteoclasts normally work in concert with osteoblasts during bone tissue remodelling. In myeloma their activity predominates and is intrinsic to disease progression. It is now clear that osteoclasts also contribute to the survival of myeloma cells but the precise mechanism(s) for this remain unresolved. As a first step, we developed a model that can support and measure osteoclast function. We showed that the osteosarcoma cell line SAOS-2 was able to secrete a calcified matrix in a fashion similar to human osteoblasts. This was deposited on the plastic substrate following treatment with 300mM ascorbic acid and 10mM ß-glycerol phosphate for 28 days. Subsequently, the cells were removed from the mineralized plates and they were used to provide a base material to measure the resorption capacity of osteoclast-like cells and investigate how myeloma cells influence that activity. We next went on to develop and characterise an in vitro osteoclastic model using the myelo-monocytic U937 cell line. Treatment with 100nM PMA and 10nM calcitriol causes these cells to merge and form multi-nucleated (Figure 1A), TRAP positive (Figure 1B) and RANK positive cells. Culturing two different myeloma cell lines, H929 and RPMI-8226, in co-culture with the osteoclast-like cells for a period of 48 hours revealed two unique sub-populations of CD138bright and CD138dim myeloma cells. Phenotypic analysis of these distinct populations showed that they expressed similar levels of CD38 but the CD138dim cells showed a significant upregulation of CD69 (p≤0.05) in both cell lines as a result of co-culture with differentiated U937 osteoclast-like cells. This data indicates that osteoclast-like U937 cells can activate a subset of myeloma cells and may provide a means of sustaining primary myeloma cells in vitro. We are currently performing RNA-seq experiments to try to understand why only a subset of cells respond to this stimulus. We will then go on to establish whether primary myeloma cells derived from patients show similar responses when co-cultured under the same conditions.
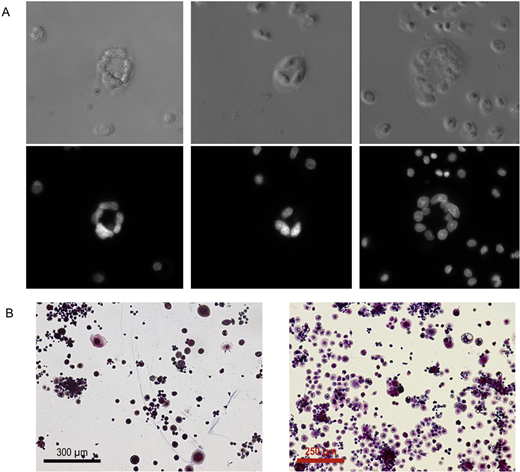
Figure 1: U937 cells have the capacity to form large multinucleated osteoclast-like cells that express tartrate resistant alkaline phosphatase (TRAP). A. Representative brightfield images coupled with images of DAPI staining following treatment with PMA and calcitriol reveals that U937 cells can become adherent and merge with one another to form large, multinucleated cells. B. Representative images of TRAP (purple) staining illustrate that these osteoclast-like U937 cells are also able to express TRAP following treatment.
Fegan:Acerta Pharma: Research Funding. Pepper:Cardiff University: Patents & Royalties: Telomere measurement patents.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal